Immunotherapeutic strategies are being utilized to harness the power of the immune system to recognize and destroy cancerous cells. However key challenges remain. While immune checkpoint inhibitor therapy can provide a more effective therapeutic option for many different advanced stage cancers; it is only effective in 30-40% of patients and nonspecific immunologic activation can lead to immune-related adverse events, including muscle weakness, joint pain, inflammatory arthritis, and myositis. In addition, immunotherapeutic strategies such as adoptive cell therapy and chimeric antigen receptors can be extremely expensive. Therefore it is imperative to develop tools that can be used to help stratify patients that would best benefit for specific types of immunotherapy, utilize model systems to study the tumor microenvironment in order to develop better combinatorial approaches, and support platforms that can aid in the identification of prognostic and predictive biomarkers.
Challenges with Immunotherapy
Role of the Tumor Microenvironment
It is known that the interactions between tumor cells and immune cells permeate the microenvironment and are dynamic and bidirectional. The concept of cancer immune surveillance proposes that the immune system is constantly surveying the host and eliminating malignant cells. However, due to genetic instability in the tumor cells and presence of immunosuppressive cells/factors some cancers are allowed to persist and eventually escape the anti-tumor immune response. Thus when tumors are palpable or reach the stage of clinical detection, these cancers have already evades the natural, intrinsic mechanisms of the host immune response. Therefore, one must focus efforts on strategies that enhance immune-mediated tumor destruction as well as restore cancer-associated immune deficiencies.
What are the sub-classes of TIME?
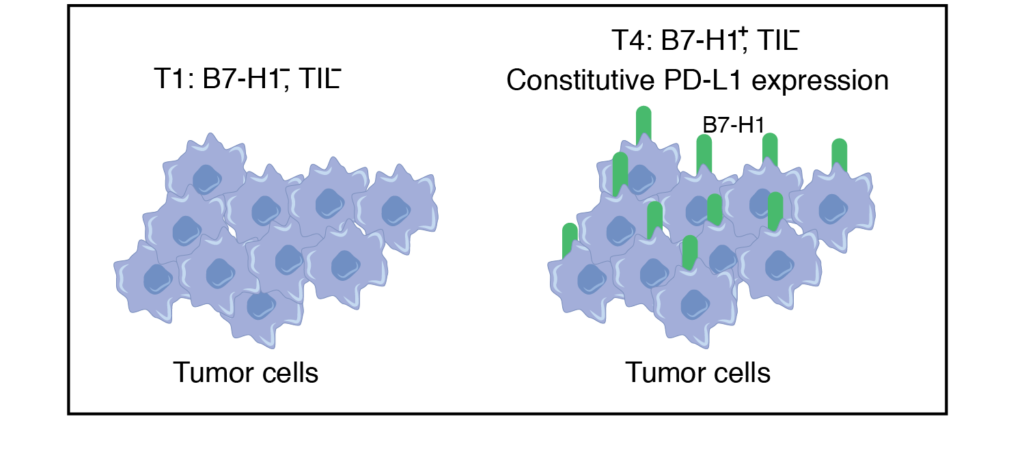
Lack of Tumor Infiltrating Lymphocytes (TIL) possibly due to factors such as tumor antigen suppression. The majority of tumors express this phenomenon.
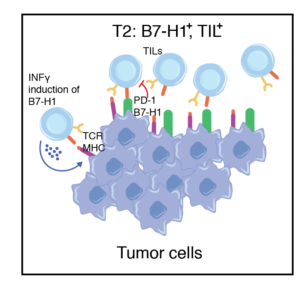
Predicted to respond to anti-PD1 therapy. Tumors containing TILs and other lymphocytes may not respond due to the expression of B7-H1 on tumors
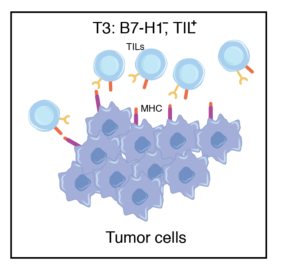
Tumors contain TILs but lack B7-H1 expression, likely owing to the absence of IFNγ production by T effector cells (Teff) and cellular dysfunction.
T4 tumors are similar to T1 except that expression of B7-H1 is present on tumors.

Modules in IO
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Immune 3D can be used to assess factors that increase immune cell migration and infiltration, reduce the production of inhibitory factors and the recruitment of suppressive cells, modulate cellular adhesion, integrins, chemokines and nutrient availability.
- Our system can be used to assess factors modulating immune cell function, such as immune checkpoints or inhibitor receptor expression.
- Spatial/ temporal relationships between immune cells, tumor cells and surrounding stroma.
- Immune 3D can be set in the presence and absence of immune cells providing a reproducible and affordable platform (compared to in vivo systems) to assess the impact of specific compounds on the tumor microenvironment and the relative contribution of the immune system.
- Importantly, the impact of specific compounds on human tumors and human immune cells can be observed and characterized.
- Immune 3D can be used in combination with immune suppressive agents such as chemotherapy, pharmacological inhibitors, as well as with cells that have been genetically manipulated in order to elucidate the mechanisms impacting anti-tumor immunity.
- Our system provides a novel platform to assess synthetic lethality and the impact of targeting defective DNA repair in cancer cells on anti-tumor immunity.

