IMMUNE 3D ® is a unique and proprietary platform that is designed to capture the core cell types to effectively capture the extracellular matrix. The platform was initially designed to naturally observe the migration patterns of immune cells and to interrogate the stromal environments that cells need to permeate to reach their destinations. Over the last few years, we have applied our understanding of the role that immune cells play in various diseases into developing 3D models that capture the disease biology in an immune context.
Platform Capabilities
Patient-derived cells or tumors are co-cultured with extracellular components such as fibroblasts and collagen. Lymphocytes (autologous or allogenic) are added to the platform and migration is observed at various time points enabling the study of important cell-cell interactions
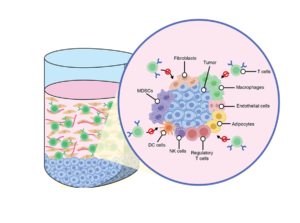
Study the contribution of immunosuppressive cells such as Regulatory T cell (Tregs), Myeloid Derived Suppressor Cells (MDSCs) and Macrophages towards immune dysfunction
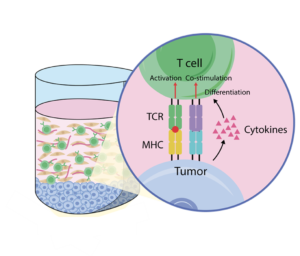
Study receptor-ligand interactions of immune cells under disease based conditions
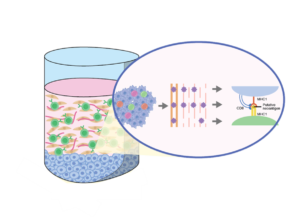
Cancers often rapidly mutate and mutations in the genome can cause these malignant cells to express mutant proteins that are tumor specific and not expressed on normal cells, referred to as neoantigens. IMMUNE 3D® can be used to uniquely expand tumor-specific T cell populations that respond to novel neoantigens.
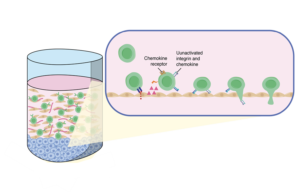
Chemokines are known for their ability to modulate lymphocyte recruitment and migration across diseases.
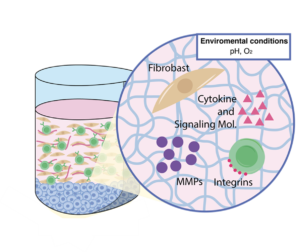
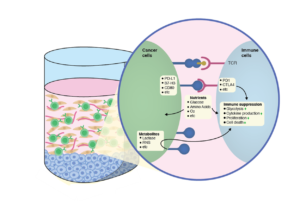
The disease environments are composed of surrounding stroma, which includes fibroblasts, endothelial cells, and immune cells, as well as the extracellular matrix. These cells contribute towards immune profile changes. Studying these interactions can lead to better therapies or biomarkers
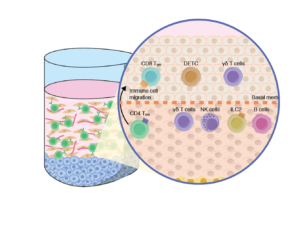
Study factors such as role of immune cell migration such as Tregs, skin gamma-delta (γδ) T cells and their role in oncology and other diseases, Innate Lymphoid Cells (ILCs) and microbial interactions and the evolution of the immune response to aged matrix
Study immunometabolism factors such as lactic acid and ROS can be measured in the presence and absence of treatment
Lymphocyte Activation
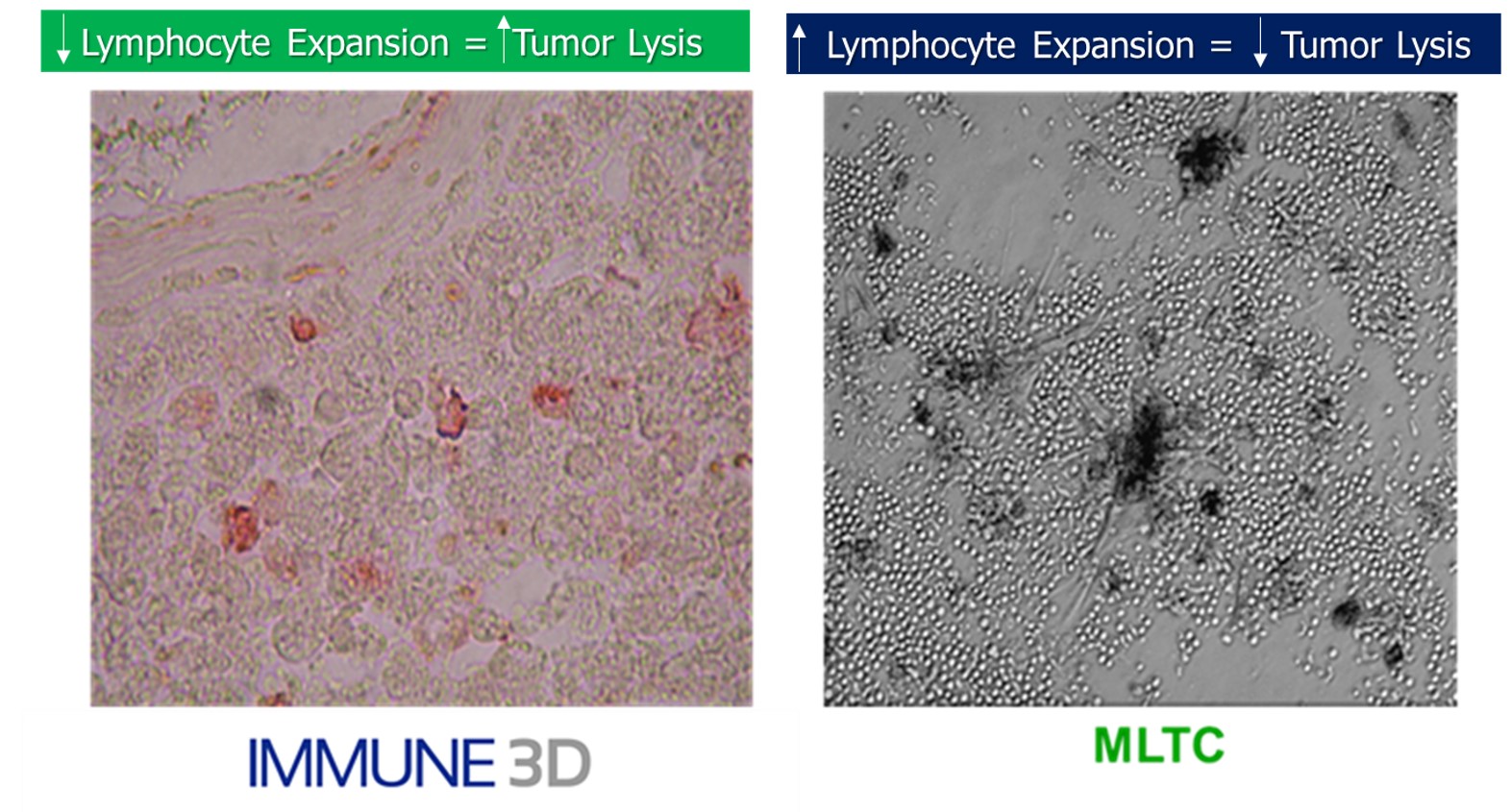
The platform design enables the selective migration of T cells resulting in specific tumor lysis
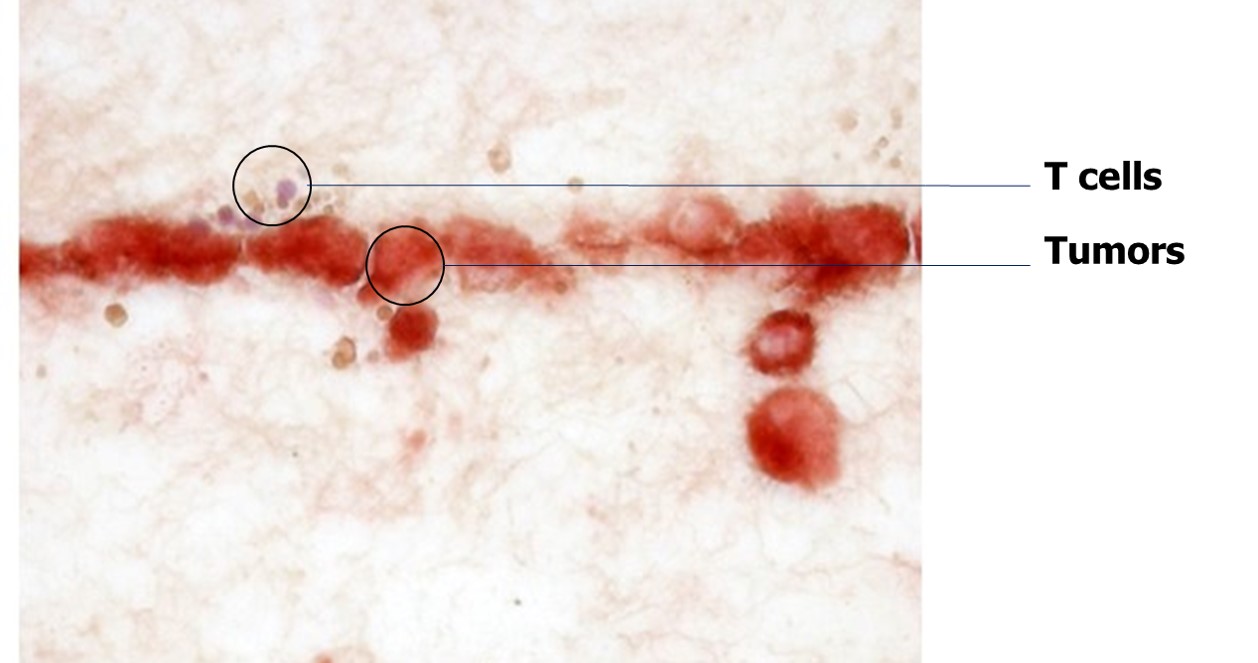
Cross section imaging
Unique and patented design enables cross sectioning of the culture to observe and quantify the migration of T cells (and other immune cells) and apoptosis of tumors in response to drug treatment. This design lends itself to study the “hot” and “cold” tumor phenomenon in cancer immunotherapy.
T cell kinetics
Compared to other 3D platforms and pre-clinical systems, IMMUNE 3D is designed to monitor and measure the kinetics of T cells and lymphocytes.
Stromal Factors
The addition of human collagen and fibroblasts enables the study of the tumor stromal components involved in T cell migration.
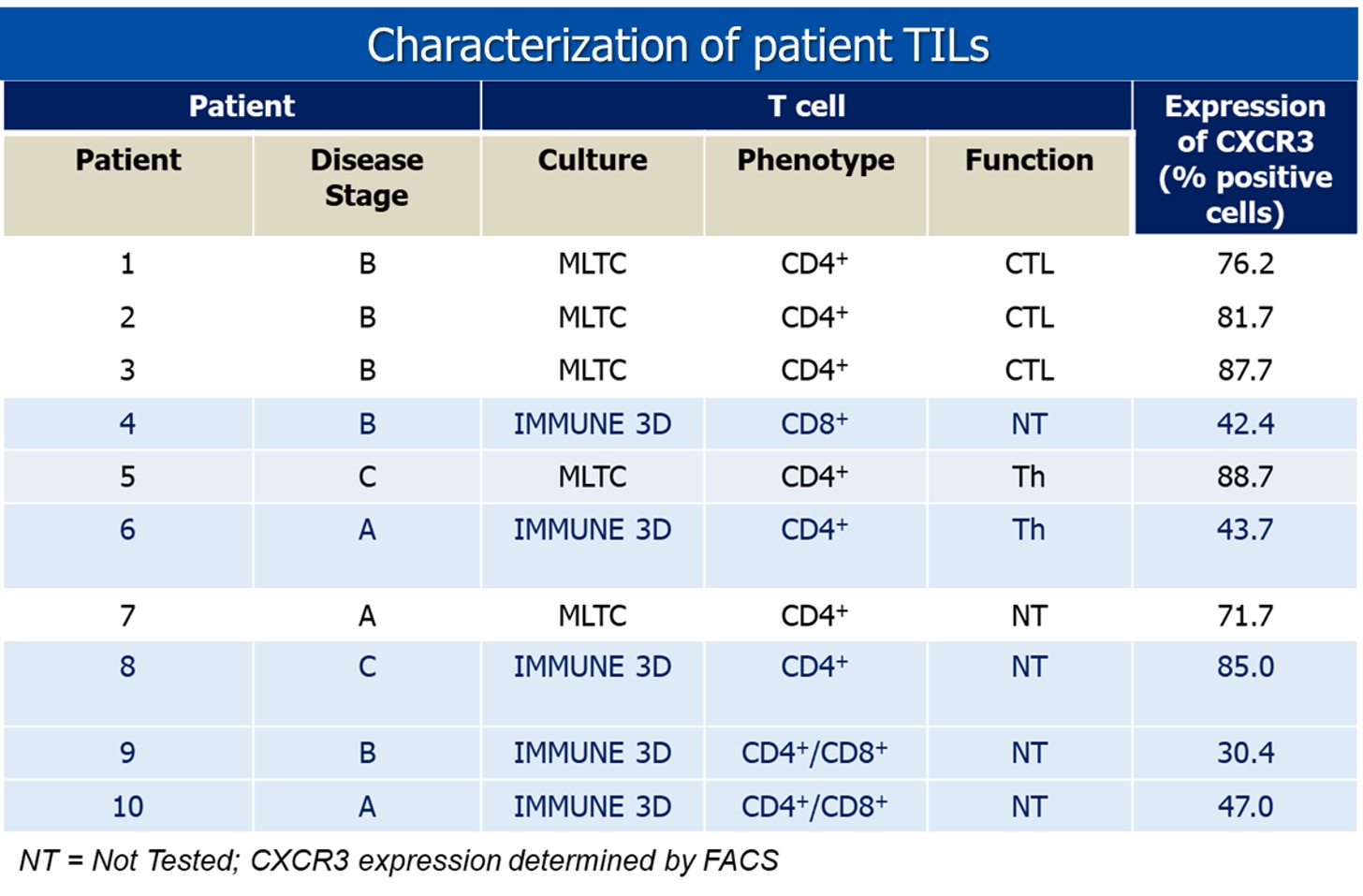
Characterization of Tumor Infiltrating Lymphocytes (TILS)
The platform has been used to characterize patient derived TILs. This is highly valuable in isolating TCRs and characterizing specific immune cell populations of interest. Here we show an example of characterization of TILs for chemokines.
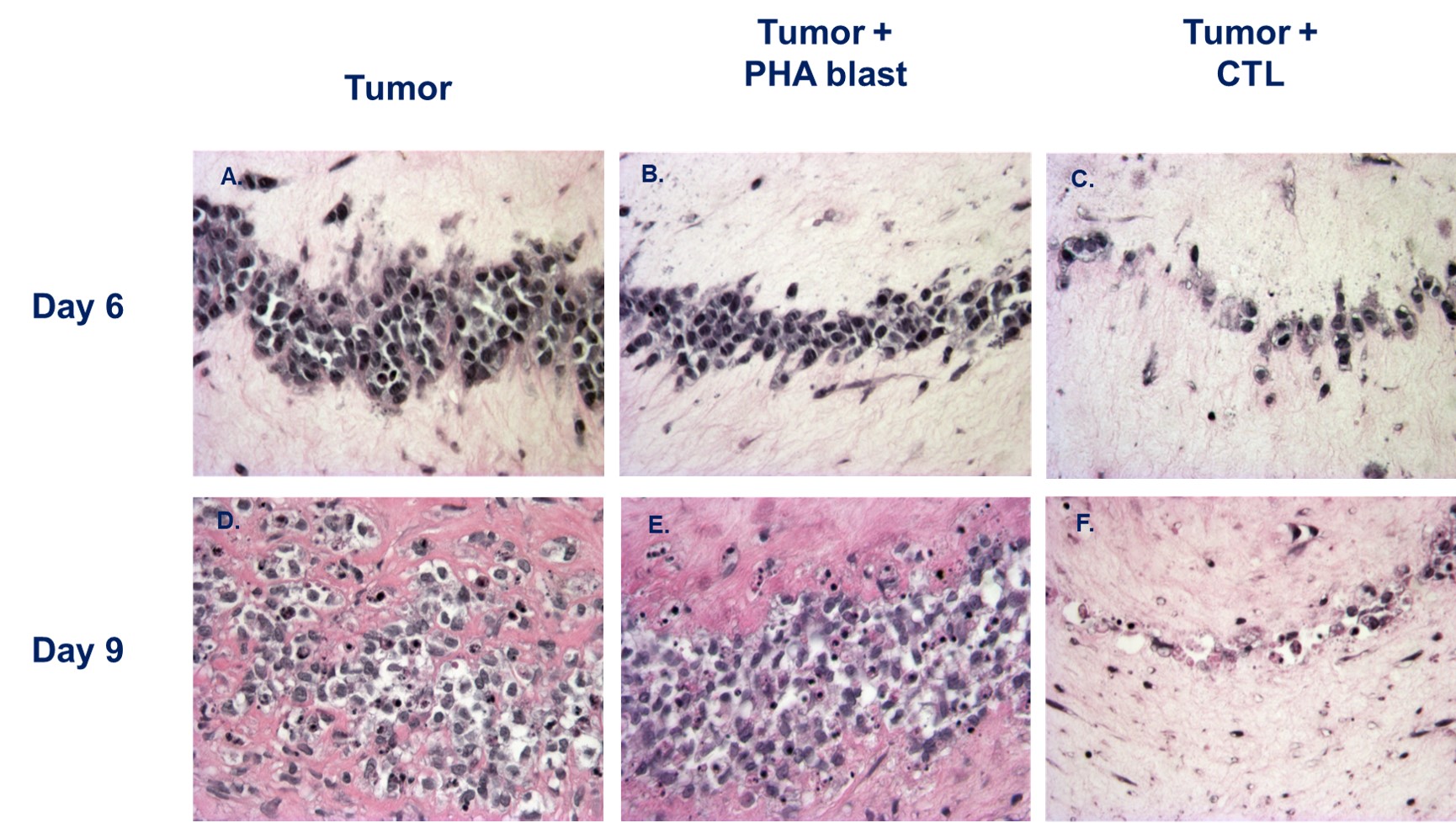
Tumor lysis imaging
Monitor the time course of tumor lysis in response to drug treatment over several days.
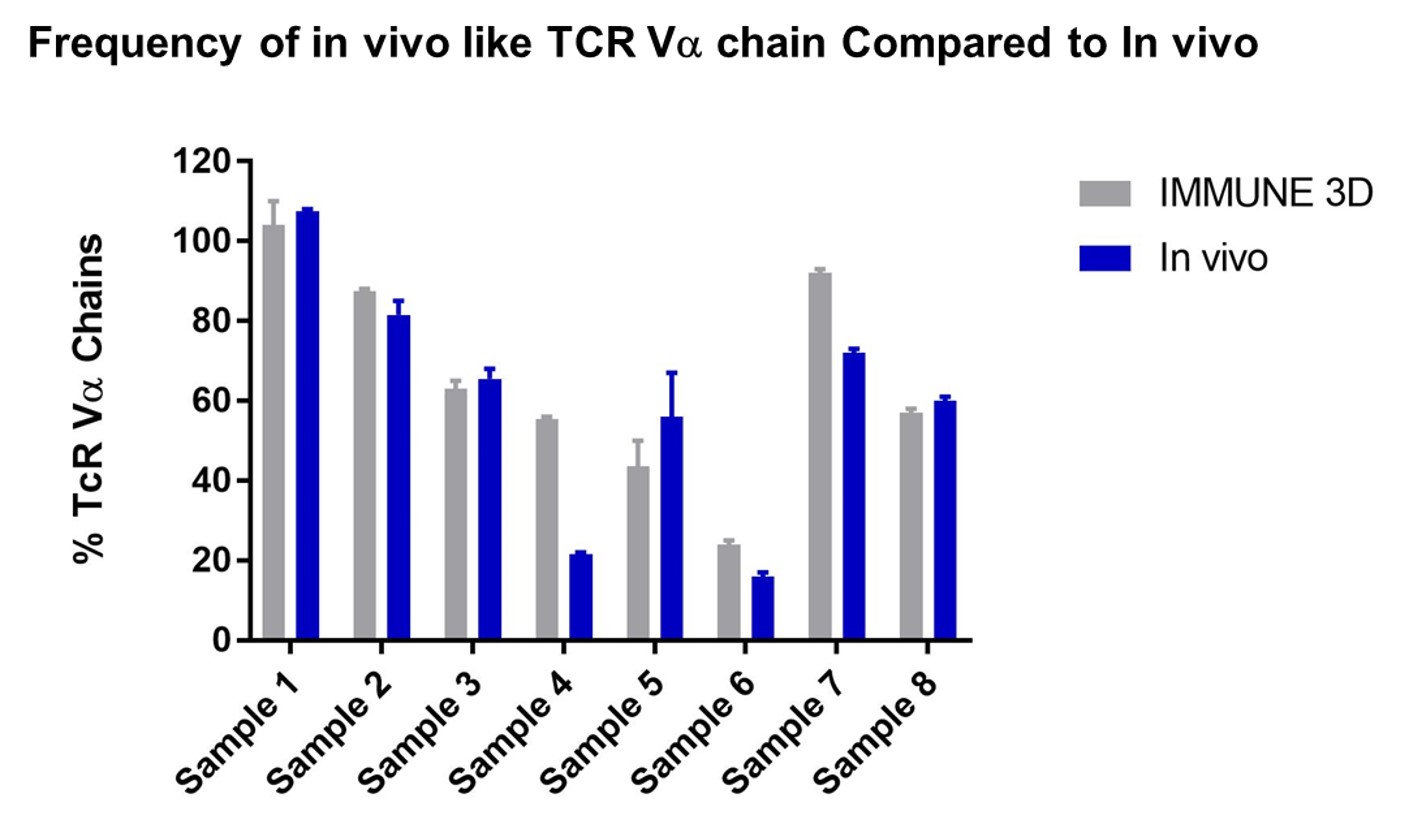
T Cell Receptor (TcR) Chains
Comparable TCRs chain preservation compared to in vivo makes it ideal for screening drug candidates in compliment to animal models
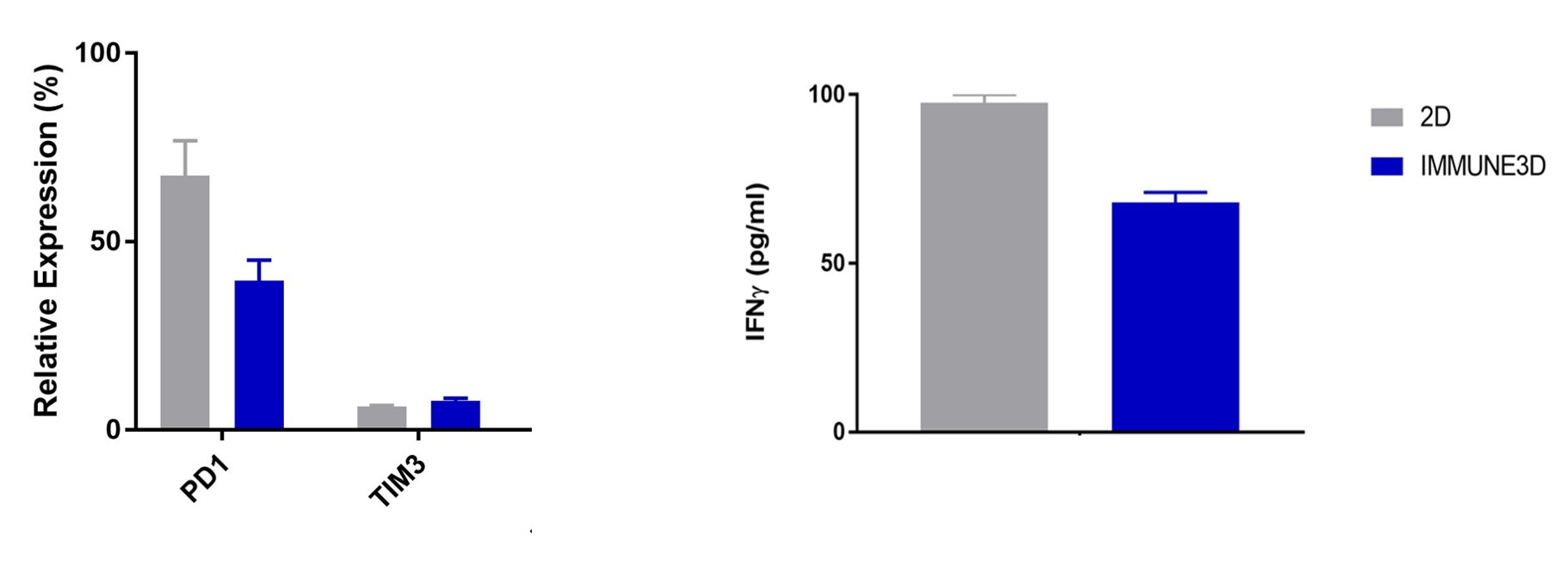
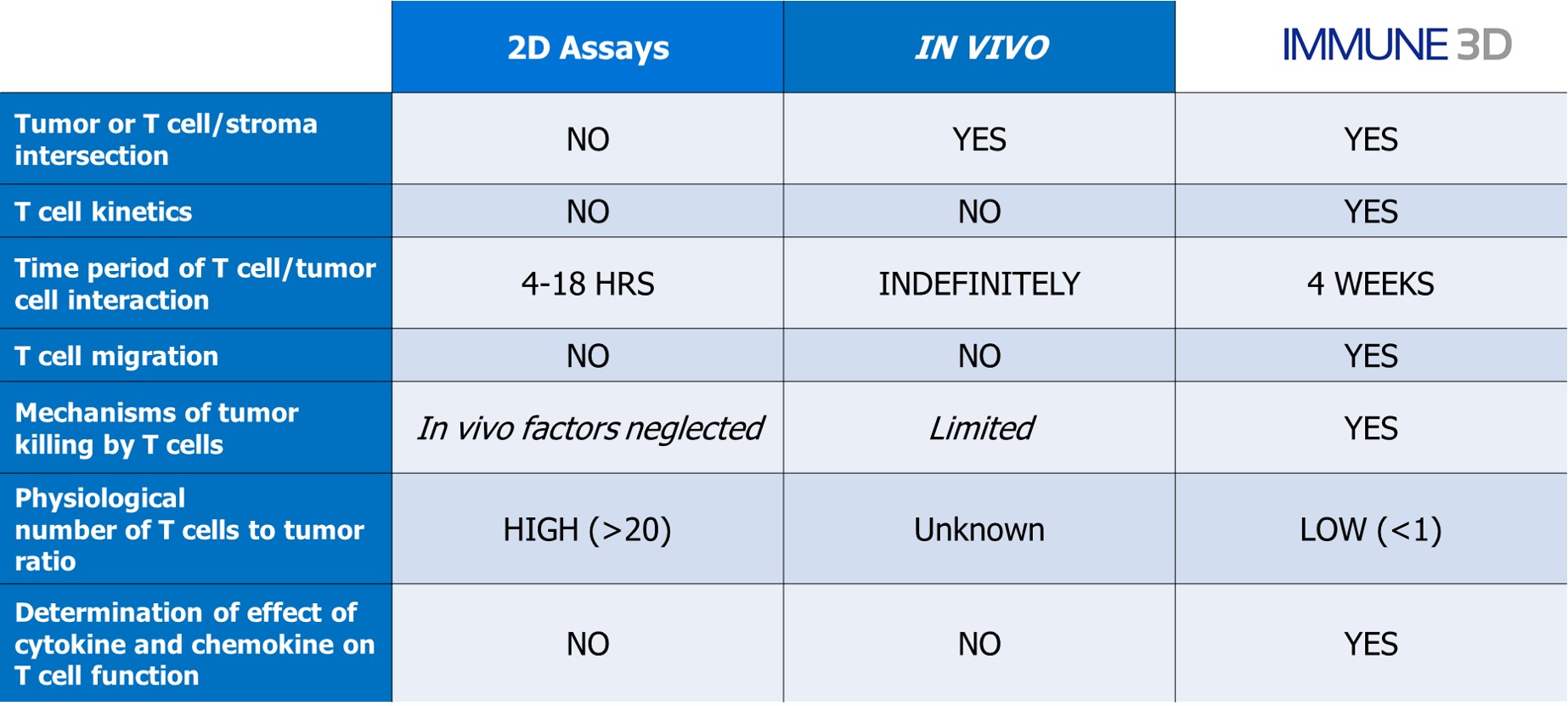
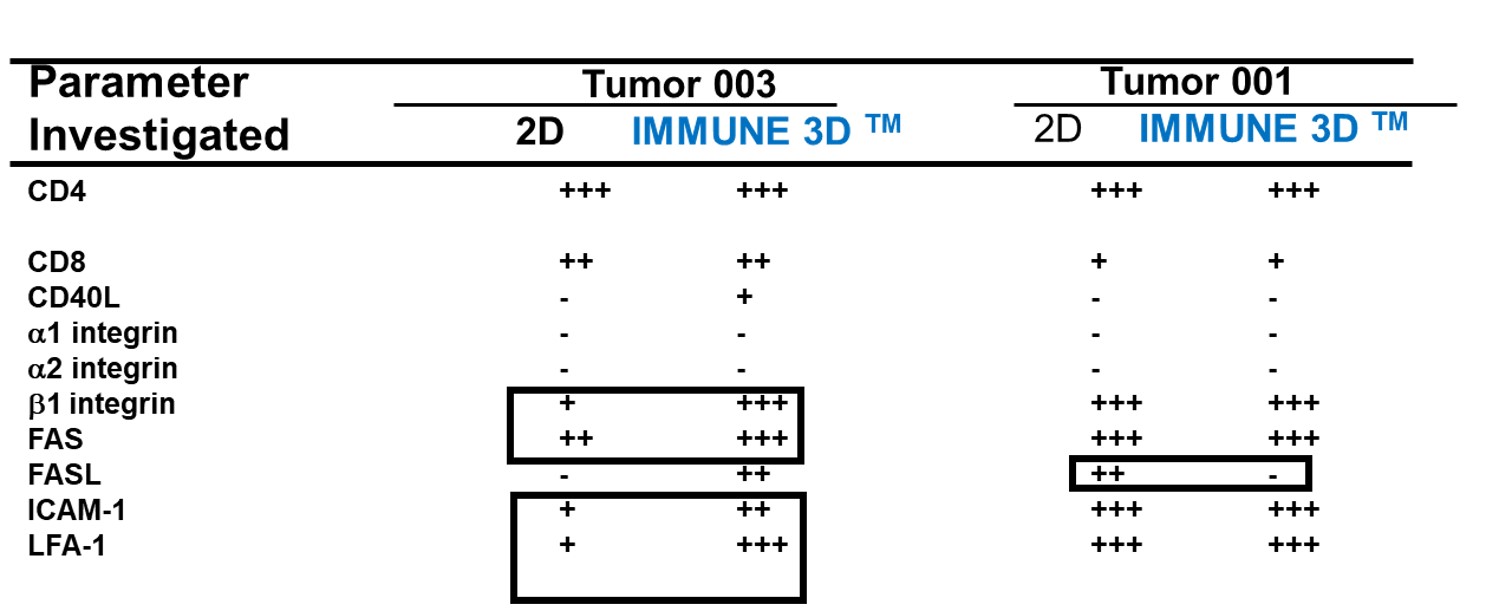
Comparison vs. 2D cultures
The acceptance of the tumor microenvironment (TME) playing a key role in cancer immunotherapy, IMMUNE 3D is well suited to capture the TME components. The inclusion of stromal components impacts cytokine and cell surface expression patterns.