TIL 2164 and Tumor 2164 is an autologous pairing obtained from a patient that did not respond to Keytruda (anti-PD1) treatment. We have extensively optimized the culture conditions and characterized the TIL and tumor for select expression markers.
Assay Description
TIL 2164 and Tumor 2164 is an autologous pairing obtained from a patient that did not respond to Keytruda (anti-PD1) treatment. We have extensively optimized the culture conditions and characterized the TIL and tumor for select expression markers.
Study Design
This assay is designed to measure the effect of test drug candidates on TIL 2164 and Tumor 2164 in comparison with Keytruda (anti-PD1). This assay will be run in 24 well plate for a 4-day co-culture period. The assay will measure the effect of test drug candidates on the following:
- TIL migration
- Cytokine responses (Multiplex of 13 cytokines)
- Quantification of apoptosis
Study Arms
- TIL 2164 + Tumor 2164 (No Compound)
- Tumor 2164
- TIL 2164 + Tumor 2164 +DMSO
- TIL 2164 + Tumor 2164 + Keytruda (anti-PD1)
The assay will be run in duplicates (2)
Deliverables
- Raw data for flow cytometry (.fcs files) – client must have Flowjo or other software to read files
- Raw data of multiplex cytokines
- Image sections (one per well)
- Powerpoint presentation of the data
Reference Study using Keytruda (anti-PD1)
TIL infiltration is a prognostic indicator to the response to immunotherapy treatment. In this study we sought to measure the effects of a test compound on TIL 2164 migration, changes to checkpoint expression patterns and apoptosis of autologous tumor 2164. TIL 2164 and Tumor 2164 were co-cultured in IMMUNE 3D. Test compound (doses: 10µm, 1µm, 0.1µm) was and Keytruda (anti-PD1) were added to designated wells. The study was conducted over 4 days.
TIL 2164 was labelled (green dye) and Tumor 2164 (blue dye) and sections of IMMUNE 3D were taken on Day 4. Each well was formalin fixed and processed for imaging. Fluorescent, H&E and TUNNEL images were stained (images not shown)
Multiplex cytokines
A multiplex cytokine assay consisting of 13 cytokines was measured from supernatants collected at Day 4. Variations in cytokine responses were observed in Test Compound (doses 10um, 1um and 0.1um) compared to Keytruda (anti-PD1). Other cytokines included but not shown were: TNFα, IL-13, IL-17B, IL-9
.
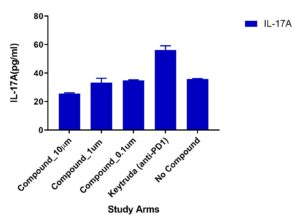
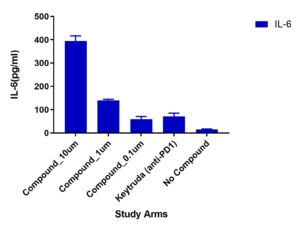
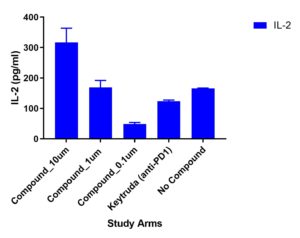
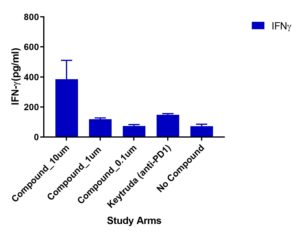
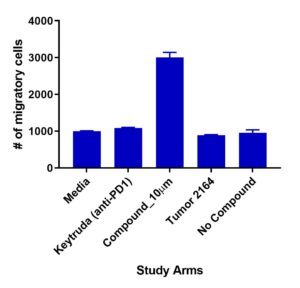
Migration of TILs
Test compound (10µm) demonstrated increased TIL 2164 migration compared to Keytruda (anti-PD1)
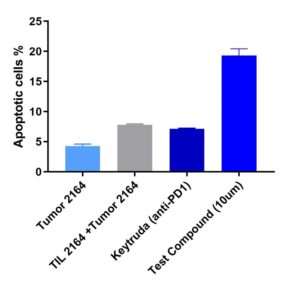
Apoptosis of Tumor
Test compound (@10µm dosed) demonstrated enhanced Tumor 2164 lysis compared to Keytruda (anti-PD1)
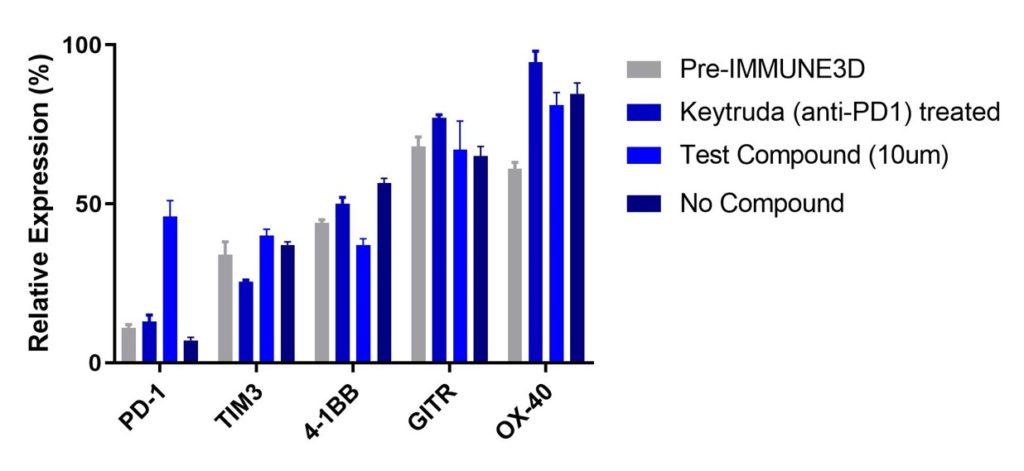
Checkpoint marker expression
We monitored the changes to key checkpoint markers pre-IMMUNE 3D (before adding the TIL and Tumor 2164 to co-culture) and after 4 days
Notes for Clients
- Minimum of 2 test compounds must be submitted
- Each dose is run in duplicates (e.g. if one test compound has 3 doses = 6 wells will be used)
- Keytruda (anti-PD1) control at 10ug/ml
- Client must provide dose and handling instructions at time of delivery of drug candidates
- If no dilution or concentration buffer provided, we will assume the use of DMSO as thed dilution of buffer
- If Client is a member of Scientist.com, Purchase Order (PO) must be submitted via Scientist.com
- Deliverables include – raw data files for cytokines and .fcs for flow cytometry
- Price includes one (1) round of revision within 5 days from receipt of data. If more time and additional rounds of revision are needed, please contact us – info@immune3d.com
- A 30% upfront fee of the PO is required to secure a position
- Cancellations are not accepted once the drug candidate is received in house
- DEADLINE FOR COMPOUND RECEIPT IS 5PM EST on JAN 7, 2020

